TPE for Blow-Fill-Seal Technology
A unique material solution for pharmaceutical packaging using the blow-fill-seal technology.
We developed a material solution for pharmaceutical packaging that opens up completely new possibilities compared to conventional solutions, particularly in the areas of usability and haptics.
The new thermoplastic elastomer (TPE) meets the changing demands of society: TPEs with soft, pleasant haptics, excellent usability, which can be sterilized at 121°C using the superheated steam process which retain their high transparency and mechanical properties even after sterilization. In addition, the TPEs have certified co-recyclability in the PP and HDPE waste streams.

With this material, we meet the previously unmet demands of the market to combine the flexibility of a polyethylene with the autoclavability at elevated temperatures of a polypropylene while maintaining transparency. These new products offer manufacturers completely new and unprecedented opportunities to package liquids in a compliant manner, and with unprecedented benefits for the user. We would be happy to present the solution in detail with reference to your specific project.
Oliver Kluge | Market Segment Manager Medical Applications at KRAIBURG TPE
OUR KNOW-HOW – YOUR ADVANTAGE
Manufacturers using the blow-fill-seal (BFS) process profit from the new solution of KRAIBURG TPE. The materials are Medical Grade Plastics and fit into the portfolio of THERMOLAST® M compounds - thus meeting the requirements of VDI 2017. The new high-end THERMOLAST® M compounds are aimed directly at manufacturers of medical substances as well as developers of packaging systems.
OUR Partners
Rommelag: The processability of the new compounds has already been confirmed for this process by tests at Rommelag. Rommelag is one of the world's leading suppliers of blow-fill-seal technology, a process for the aseptic filling and packaging of liquids and semisolids.


Borealis contributed to the development of the new compounds, providing one of its Bormed™ medical grades as a base. As one of the world's leading providers of advanced and sustainable polyolefin solutions, Borealis partnered with KRAIBURG TPE to create a material solution that meets the changing demands of society.
WHAT DOES BLOW-FILL-SEAL MEAN?
The blow-fill-seal manufacturing process combines several steps of aseptic drug manufacturing into one system. In the first initial step, material is extruded into a mold to form a single-dose container. Next, the liquid product is filled and immediately sealed from the environment. All this is achieved in one process without human intervention.
The Blow-Fill-Seal Process at a glance - Just five steps for the finished product
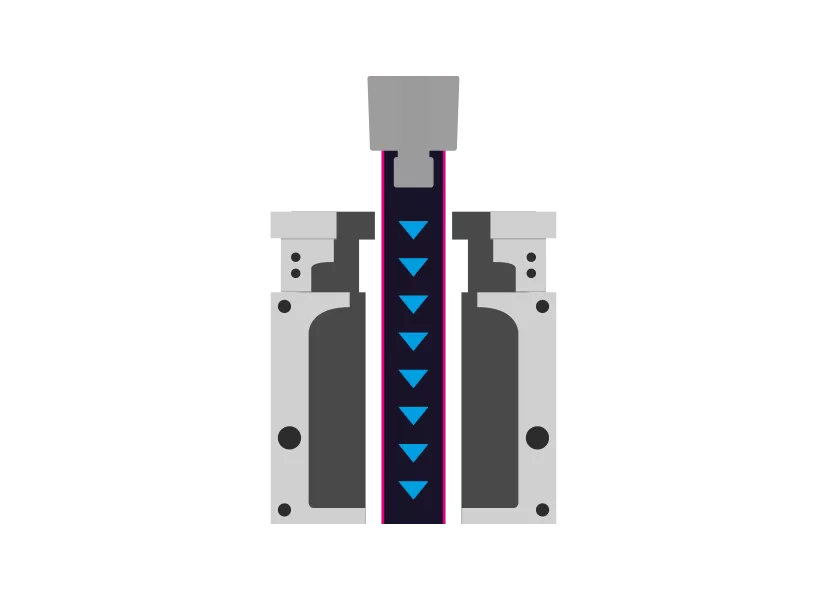
Step 1 - Extrusion
First, a tube is extruded from the TPE. This is then used by the blow molding tool.
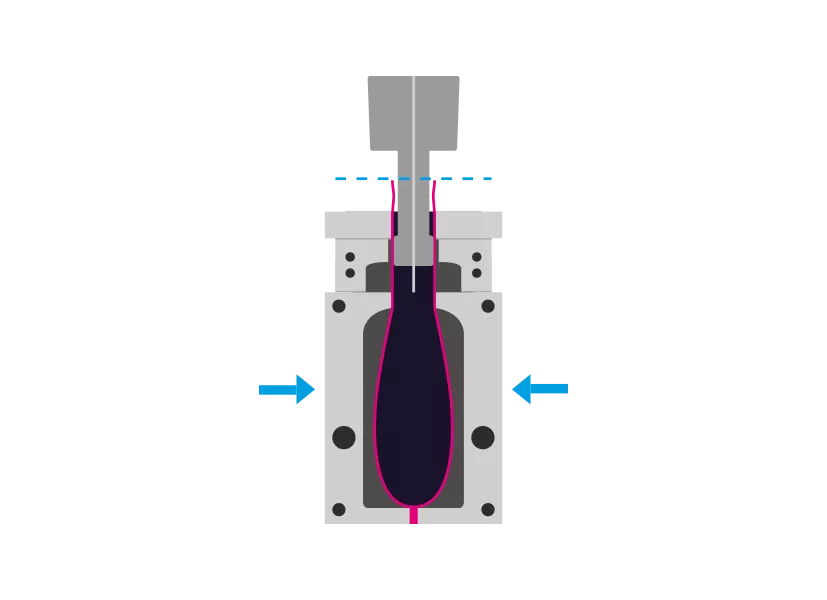
Step 2 - Molding
The tool closes, squeezes and welds the bottom of the hose. A mandrel is used to inflate the upper part of the tube to the mold. Additionally, vacuum can be used.
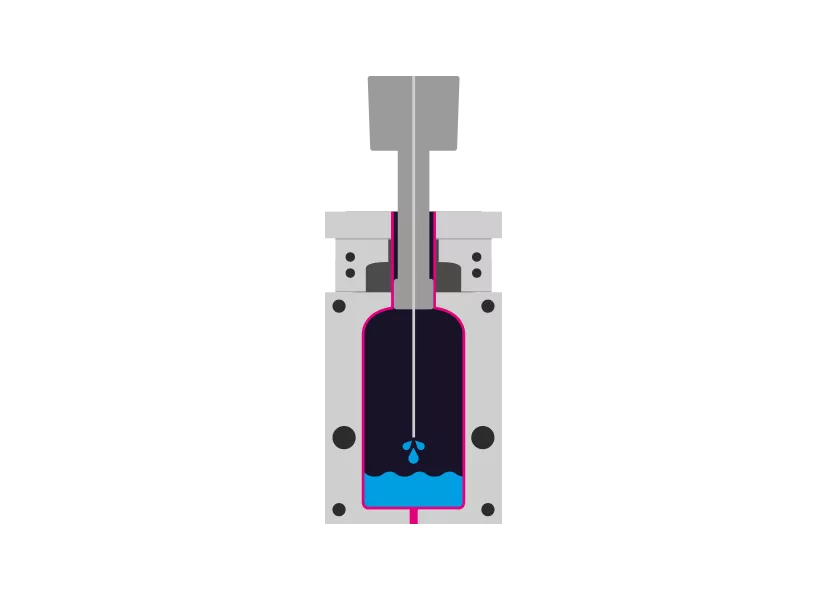
Step 3 - Filling
The filling also enters the mold aseptically via the mandrel.
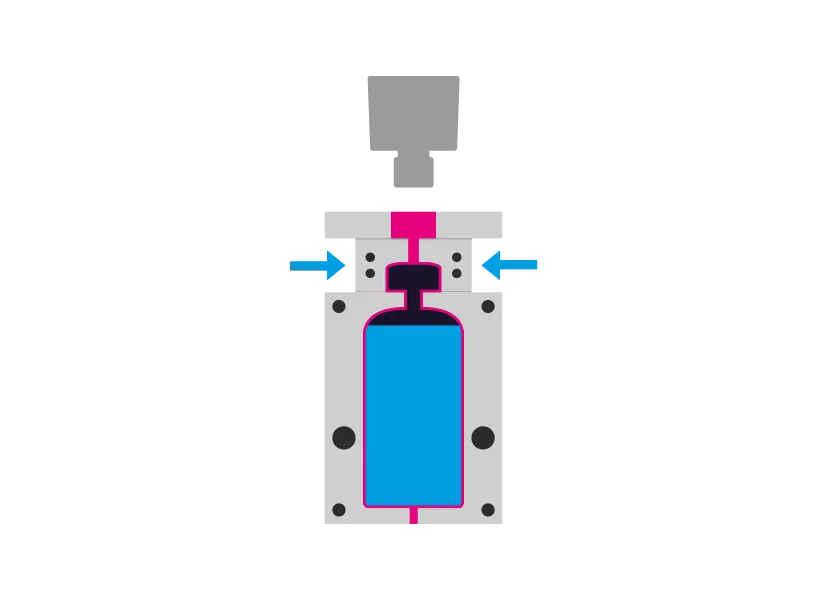
Step 4 - Sealing
The mandrel is removed. The container is receives the desired closure system and is sealed aseptically.
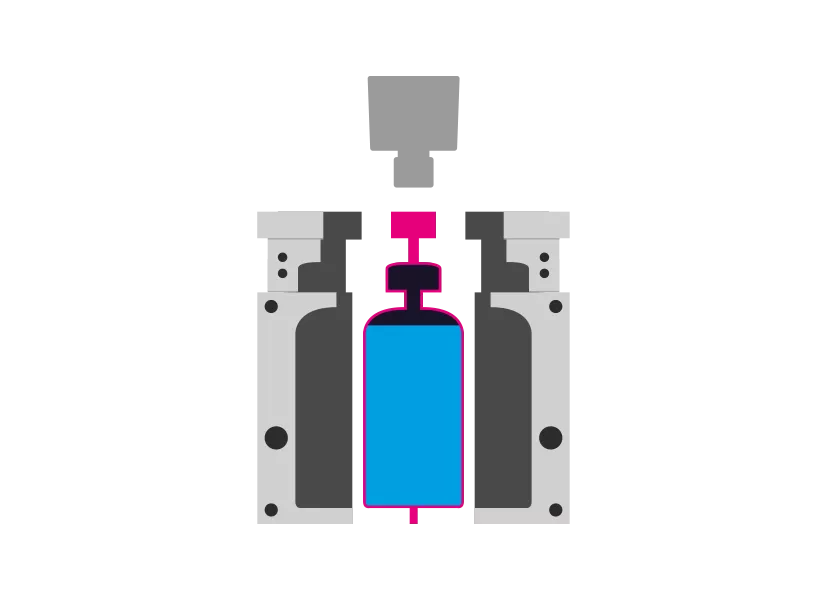
Step 5 - Demolding
The mold opens and the product is removed. The process starts again.
Contact us!
Dominik Hilz
Market Segment Manager Medical Applications

Phone: +49 8638 9810-391
E-Mail: dominik.hilz@kraiburg-tpe.com
Oliver Kluge
Market Segment Manager Medical Applications

Phone: +49 8638 9810-479
E-Mail: oliver.kluge@kraiburg-tpe.com
DISCOVER MORE
THERMOLAST® M
THERMOLAST® H
Medical TPE

